Professor Na Gun and Kang Min-ho's team developed copper ion detection technology in vivo using...
- Writer :External Affairs Team
- Date :2025.04.30
- Views :44
Professor Na Gun and Kang Min-ho's team developed copper ion detection technology in vivo using sulfur doping carbon points
- Real-time detection of copper ion concentration in vivo without expensive equipment and complex pretreatment with sulfur-doped carbon points
- Research results are published in the famous international academic journal 'Small (IF=13.0)' in the micro and nano fields
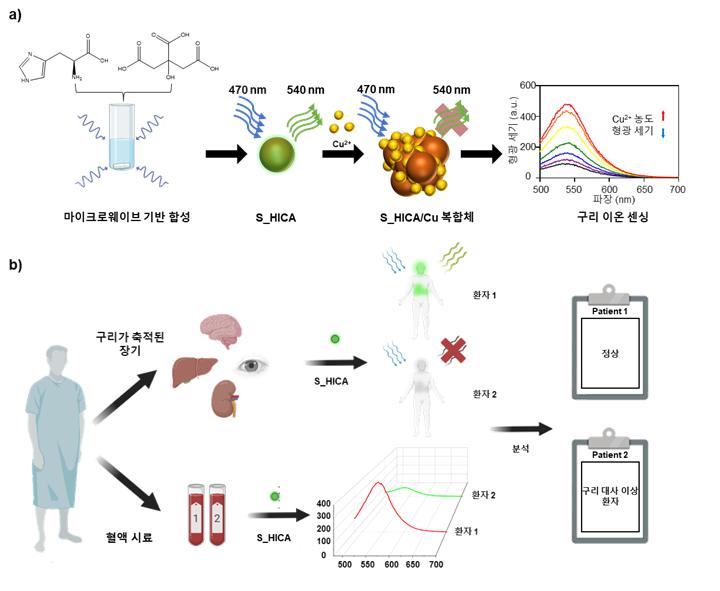
△ Conceptual diagram of copper ion detection using sulfur-doped carbon points (S_HICA)
A research team led by Professor Na Gun and Kang Min-ho of The Catholic University of Korea's Biomedical Chemical Engineering Department developed a technology to effectively detect copper ion concentration in living organisms by using "sulfur doping carbon point" in collaboration with Professor Jeong Hyeon-do of Hanyang University's New Material Engineering Department.
The results of the study were published in "Small (IF=13.0)," a famous international academic journal in the micro and nano fields, and Professor Na Gun of the Department of Biomedical and Chemical Engineering at Catholic University, Professor Kang Min-ho, and Professor Chung Hyeon-do of the Department of New Materials Engineering at Hanyang University participated as co-corresponding authors, and Dr. Jin Min-young of the Department of Biotechnology at Catholic University participated as the first author.
Copper ions accumulated in the body can cause various diseases such as Wilson's disease, Menkes syndrome, and Parkinson's disease depending on the concentration, so it is important to accurately detect them. Existing copper ion detection methods have several limitations, such as a complicated pre-processing process, high measurement time and cost, and interference problems caused by other materials.
To solve this problem, the research team developed "Sulfur-doped carbon dots (S_HICA)," a nanomaterial that specifically recognizes copper ions and has high sensitivity. Carbon dots are nano-sized carbon-based materials with excellent fluorescence characteristics and high biocompatibility, which have high potential use in the bio field. The sulfur-doped carbon dots developed by the research team can measure copper ion concentration according to changes in fluorescence intensity, can be easily manufactured through microwave synthesis, and provide reliable results even within a wide pH range (1-11) and a wide range of temperatures (20-80℃).
As a result of the study, it was confirmed that the developed carbon point has high fluorescence efficiency and short reaction time with copper ions, enabling real-time copper ion measurement in various living environments such as cell, blood, and animal models. In addition, high-performance equipment such as ultra-low temperature transmission electron microscopy found that as the carbon point combines with copper ions to form a composite, static quenching occurs, which decreases the fluorescence intensity of the carbon point.
"This study presents a simple and fast diagnostic means that can replace existing expensive equipment and complex preprocessing processes," said Professor Na Gun of the Department of Biomedical Chemical Engineering at The Catholic University of Korea. "It is expected to contribute to the commercialization and various applications of copper ion detection technology."

(image 1) It shows a static quenching phenomenon of fluorescence in which the fluorescence intensity of the carbon point decreases as nano-sized sulfur-doped carbon points form a complex with copper ions. Using this, copper ions can be quickly detected in organs or blood where copper is accumulated in the body, so it can be applied to a wide range of fields such as diagnosing copper metabolic abnormalities.
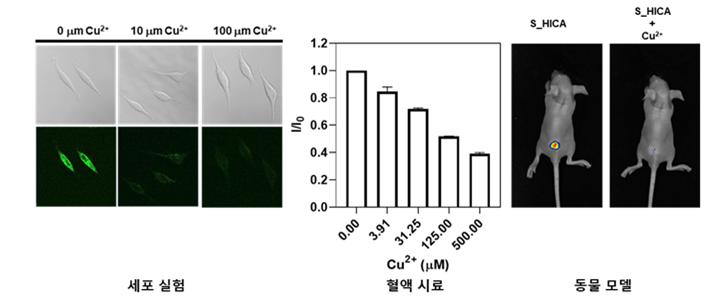
(image 2) Experiment to detect copper ions in cell, blood, and animal models using sulfur-doped carbon points (S_HICA). It was confirmed in cell, blood, and animal models that the fluorescence intensity of the carbon point gradually decreased according to the copper ion concentration. In particular, the fluorescence distribution can be confirmed even within animal models, showing the possibility of real-time copper ion detection.

