-
The Catholic University of Korea Professor Lee Hyeok's joint research team identifies the early progression mechanism ofAuthor : 관리자Date : 2022.10.06Hit : 369
-

The Catholic University of Korea Medical School Professor Lee Hyeok's joint research team
identifies the early progression mechanism of aortic valve disease caused by hyperlipidemia
- Confirms the effect of reducing inflammation through the regulation of serum LDL cholesterol and PPARγ expression
- Expected to be used for understanding the mechanism of aortic valve disease occurrence and development of therapeutics
- Published in "Nature Communications (IF 17.694)," an internationally renowned academic journal
Professor Hyeok Lee of the Department of Microbiology, College of Medicine, the Catholic University of Korea, conducted joint research with Professor Choi Jaehoon of the Department of Biotechnology, Hanyang University, and redefined the early progression mechanism of aortic valve disease caused by hyperlipidemia based on the single-cell transcriptome analysis.
Aortic valve disease can be caused by endothelial cell damage or lipid deposition in the aortic valve due to hyperlipidemia, reducing the valve due to irreciprocal changes accumulated by fibrosis and calcification as the disease advance. In the end, the valve fails to open when needed and advances to aortic stenosis, the state that the left ventricle fails to circulate blood to the aorta.According to previous studies, aortic valve stenosis is a dangerous disease with a 50% survival rate within 2 years if symptoms appear. However, only invasive treatment methods such as valve replacement exist, and treatment using drugs is still insufficient. Therefore, it was important to approach from a preventive point of view and identify the biological mechanism for the initial formation and progression of aortic valve disease.

The Catholic University of Korea Medical School Professor Lee Hyeok
Professor Lee Hyeok's joint research team used the "single-cell transcriptome analysis" method, which has recently been in the limelight, to identify the initial mechanism. After separating aortic valves from normal or hyperlipidemic mice into single cells, single-cell RNA sequencing revealed the types of immune cells that are mainly increased in the hyperlipidemic valves based on the mRNA expression of each cell.In addition, aortic valvular endothelial cells, which are specialized in lipid handling, increase in hyperlipidemia, and the corresponding endothelial cells exhibit anti-inflammatory effects through the expression of the transcription factor "PPARγ (Peroxisome Proliferator-Activated Receptor)."
The joint research team confirmed cell diversity and changes in the aortic valve under normal or hyperlipidemia conditions and found that control of serum LDL cholesterol and PPARγ activity alleviated intravalvular inflammation in early aortic valve disease. The results of this study are expected to be used for understanding the mechanism of early aortic valve disease and disease prevention using hyperlipidemia drugs and PPARγ agonists.
Professor Lee Hyeok said, "This study suggests the results of disease mechanisms and prevention methods by applying the latest genome analysis technology. It can also be an exemplary joint research case of technology-disease research teams."
Meanwhile, this study was participated by Professor Lee Hyeok and Dr. Kim Nayoung of the Department of Microbiology, College of Medicine, The Catholic University of Korea. The research, "Single-cell transcriptomics reveal cellular diversity of aortic valve and the immunomodulation by PPARγ during hyperlipidemia," was published online on September 17 in the world-renowned journal Nature Communications (IF 17.694). (The end) (includes reference material and photos).
[Reference]
Reference 1. Results of single cell transcriptome analysis on mouse aortic valve cells
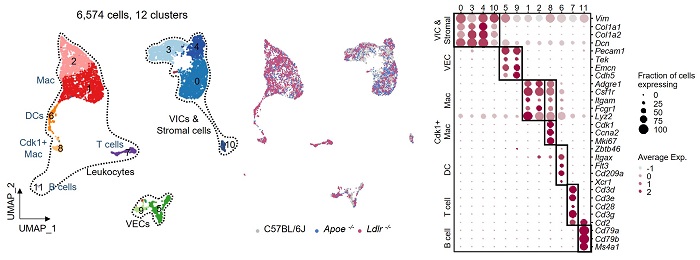
[Reference 1. Description]
In order to investigate the cell dynamics related to hyperlipidemia in the aortic valve, a single-cell transcriptome analysis was performed on the aortic valve of a mouse. As a result, 6,574 cells were classified into 12 cell clusters, and each cell cluster was classified into three cell systems: VIC, VEC, and leukocyte (macrophages, DCs, T cells, and B cells), according to the level of gene expression.
Reference 2. Increased lipid deposition and macrophage infiltration in the aortic valve according to the increase in blood LDL cholesterol
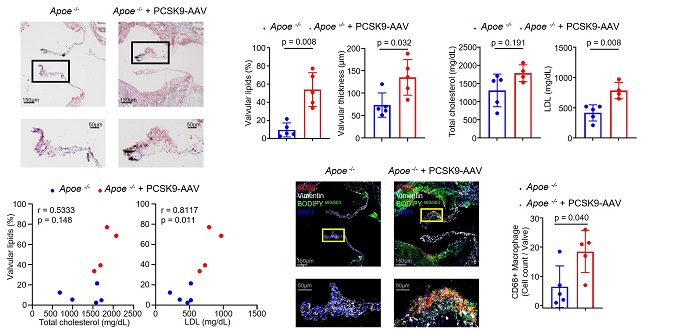
[Reference 2. Description]
Mice injected with PCSK9-AAV to induce an increase in blood LDL cholesterol had 5.7 times more lipid deposition (Oil Red O staining image) than the control group (Apoe-/- mice), and found that macrophages increased. Through this, it was confirmed that an increase in serum LDL cholesterol affects aortic valve lesions by inducing lipid deposition and macrophage infiltration in the aortic valve.
Reference 3. Whole-mount immunofluorescence staining image to visualize markers of immune cells present in the aortic valve
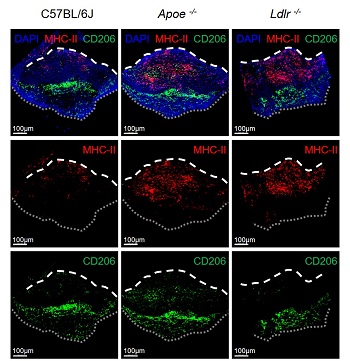
[Reference 3. Description]
Through whole-mount immunofluorescence staining, it was confirmed that macrophages expressing MHC-II (red) and CD206 (green), which are mainly present in the aortic valve, are spatially located differently.
Reference 4. Results of flow cytometry showing reduction of inflammation in mouse aortic valve by administration of transcription factor PPARγ agonist (pioglitazone hydrochloride)
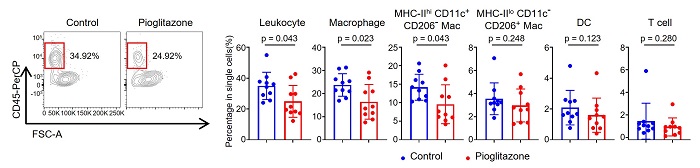
[Reference 4. Description]
When a PPARγ agonist was administered to hyperlipidemic mice, the percentage of aortic valve leukocytes decreased significantly compared to the control group. MHC-IIloCD11c-CD206+ macrophages, DC, and T cells did not show significant changes.
Reference 5. Increased expression of inflammation-related genes by PPARG gene silencing in patient-derived aortic valve endothelial cells
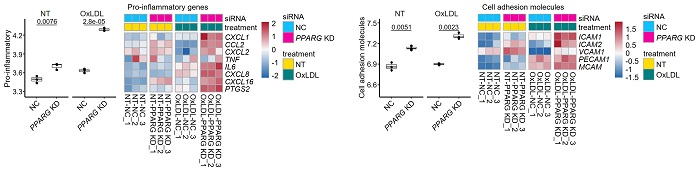
[Reference 5. Description]
It was identified that PPARG gene silencing in patient-derived aortic valve endothelial cells increased expression of inflammation-related gene such as CXCL1, CCL2, CXCL16, IL6, ICAM1, ICAM2 in VEC.
[Photo Description]
Professor Lee Hyeok, Department of Microbiology, College of Medicine, the Catholic University of Korea,
Professor Choi Jaehoon Choi, Department of Biotechnology, Hanyang University
-
Attachment File

